Acute myocardial infarction is implicated in over 30% of global mortality, annually claiming more lives than all cancers, accidents and AIDS combined. Although medical therapy using lipid-lowering statins has lowered mortality rates in the past two decades, acute coronary events continue to occur in over two-thirds of patients on statin therapy and the high incidence of AMI-related death remains a daunting reality. This ensuing residual risk has motivated efforts to develop new drugs that target additional risk factors such as inflammation to mitigate AMI. Fibrillar collagen is a key extracellular matrix macromolecule conferring mechanical strength on the fibrous cap that shields the thrombogenic necrotic core from contact with luminal blood. A stable fibrous cap is rich in collagen synthesized by vascular smooth muscle cells, which together impart mechanical integrity. Pro-inflammatory cytokines expressed by activated macrophages stimulate the production of matrix metalloproteinases (MMPs) that degrade collagen, drive the apoptosis of vSMCs and inhibit collagen synthesis. Acting together, these inflammatory processes compromise net collagen content and fiber architecture, predisposing a plaque to rupture. The mechanisms leading to plaque rupture are thus driven by a cooperative dialogue between collagen remodeling and inflammatory signaling, underscoring the fact that knowledge of plaque collagen content and architecture is crucial in advancing our understanding of VP etiology, determining drug efficacy and identifying new therapeutic targets to prevent AMI.
In collaboration with the Bouma laboratory at the Wellman Center, we are investigating and translating a new technique, polarization-sensitive optical frequency domain imaging (PS-OFDI), for intracoronary assessment of plaque collagen in patients. PS-OFDI measures changes in the polarization state of probing light to evaluate birefringence, a material property that is elevated in tissues containing proteins with an ordered structure, such as organized collagen. Therefore, beyond the visualization of microstructural features in conventional OFDI, polarization contrast in PS-OFDI provides additional information about collagen architecture within plaques and fibrous caps, where low birefringence likely indicates decreased stability (Figs. 1, 2). In birefringent tissue, light polarized along directions parallel and perpendicular to the collagen fiber orientation travels at different velocities, incurring a relative delay or phase retardation, δ. The phase retardation, δ, accumulates with depth at a rate proportional to the magnitude of birefringence, which is directly related to the thickness, density and organization of collagen fibers. To obtain a PS image, depth-resolved Stokes parameters are determined and the corresponding δ values are displayed. Further, by measuring the local depth-derivative of δ, a tomogram of tissue birefringence can be displayed to visualize collagen architecture at high spatial resolution.
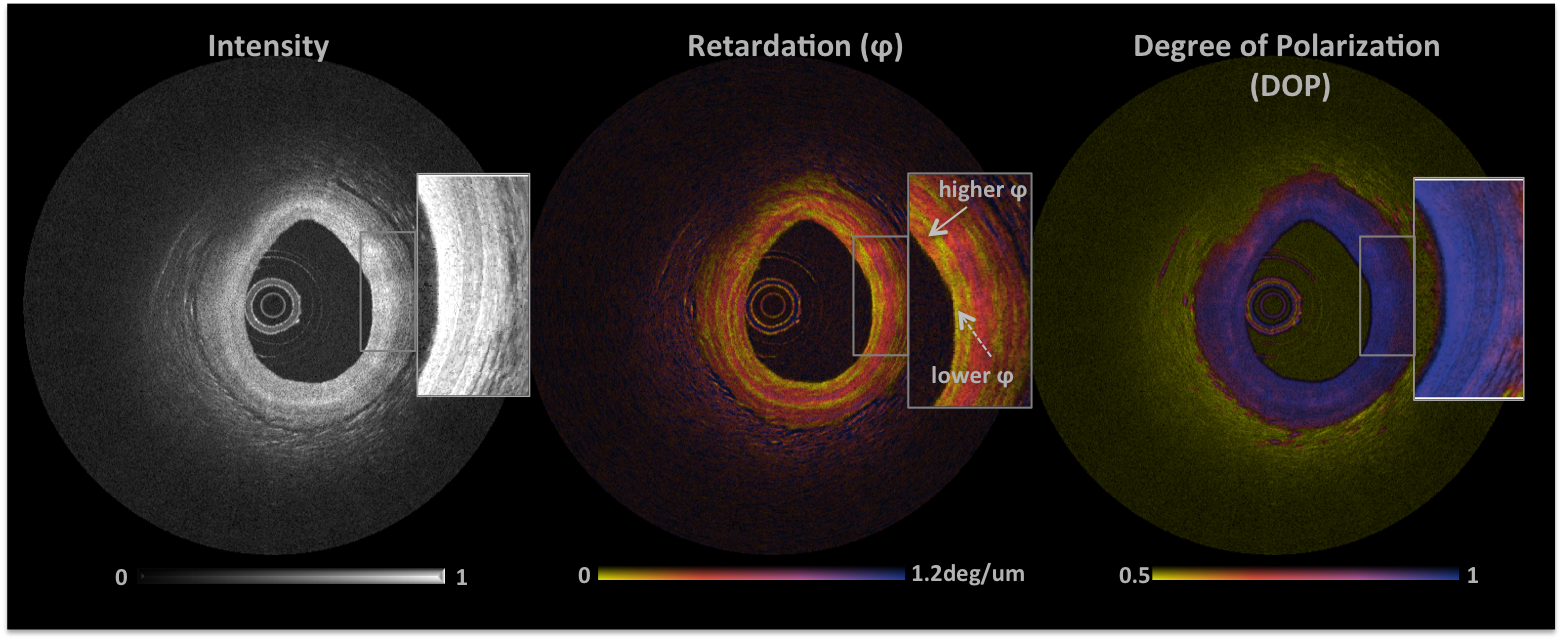
PS-OFDI image of a human coronary artery
The conventional OFDI image is seen on the left showing microstructural details of the three layers of a coronary cross-section. The corresponding retardation image is in the center with a folor bar from yellow to blue represents low to high birefringence (0-1.2°/μm). A fibrous plaque with moderate collagen is seen at 10’o’clock with a band of high birefringence within the media likely due to vascular smooth muscle cells. A degree of polarization is maintained across the arterial cross-section as shown on the right.
The image below show regions of strong birefringence correspond to the location of a fibrous plaque with thick, abundant collagen fibers.
Relevant Publications
Optimizing flushing parameters in intracoronary optical coherence tomography: an in vivo swine study. Suter MJ, Kashiwagi M, Gallagher KA, Nadkarni SK, Asanani N, Tanaka A, Conditt GB, Tellez A, Milewski K, Kaluza GL, Granada JF, Bouma BE, Tearney GJ. Int J Cardiovasc Imaging. 2015 Aug;31(6):1097-106. doi: 10.1007/s10554-015-0668-0. Epub 2015 Apr 29. PMID: 25922149
Optical measurement of arterial mechanical properties: from atherosclerotic plaque initiation to rupture. Nadkarni SK. J Biomed Opt. 2013 Dec;18(12):121507. doi: 10.1117/1.JBO.18.12.121507. Review. PMID: 24296995
Spectral binning for mitigation of polarization mode dispersion artifacts in catheter-based optical frequency domain imaging. Villiger M, Zhang EZ, Nadkarni SK, Oh WY, Vakoc BJ, Bouma BE. Opt Express. 2013 Jul 15;21(14):16353-69. doi: 10.1364/OE.21.016353. PMID: 23938487
Artifacts in polarization-sensitive optical coherence tomography caused by polarization mode dispersion. Villiger M, Zhang EZ, Nadkarni S, Oh WY, Bouma BE, Vakoc BJ. Opt Lett. 2013 Mar 15;38(6):923-5. doi: 10.1364/OL.38.000923. PMID: 23503261
Intravascular optical imaging technology for investigating the coronary artery. Suter MJ, Nadkarni SK, Weisz G, Tanaka A, Jaffer FA, Bouma BE, Tearney GJ. JACC Cardiovasc Imaging. 2011 Sep;4(9):1022-39. doi: 10.1016/j.jcmg.2011.03.020. Review. PMID: 21920342
Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Liu L, Gardecki JA, Nadkarni SK, Toussaint JD, Yagi Y, Bouma BE, Tearney GJ. Nat Med. 2011 Jul 10;17(8):1010-4. doi: 10.1038/nm.2409. PMID: 21743452
Evaluation of collagen in atherosclerotic plaques: the use of two coherent laser-based imaging methods. Nadkarni SK, Bouma BE, de Boer J, Tearney GJ. Lasers Med Sci. 2009 May;24(3):439-45. doi: 10.1007/s10103-007-0535-x. Epub 2008 Apr 2. Review. PMID: 18386093
Measurement of collagen and smooth muscle cell content in atherosclerotic plaques using polarization-sensitive optical coherence tomography. Nadkarni SK, Pierce MC, Park BH, de Boer JF, Whittaker P, Bouma BE, Bressner JE, Halpern E, Houser SL, Tearney GJ. J Am Coll Cardiol. 2007 Apr 3;49(13):1474-81. Epub 2007 Mar 21. PMID: 17397678
Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. Kawasaki M, Bouma BE, Bressner J, Houser SL, Nadkarni SK, MacNeill BD, Jang IK, Fujiwara H, Tearney GJ. J Am Coll Cardiol. 2006 Jul 4;48(1):81-8. Epub 2006 Jun 9. PMID: 16814652