Autopsy studies in patients have revealed a type of coronary plaque, the thin cap fibroatheroma (TCFA) implicated at the site of culprit thrombi in >70% of patients who have succumbed to AMI. Technologies such as optical coherence tomography (OCT), intravascular ultrasound (IVUS) and near infrared spectroscopy (NIRS) provide important microstructural and compositional information related to the risk of rupture. Yet, the prediction of plaque rupture leading to AMI remains an elusive goal. This is because plaques with similar vulnerable morphologic features do not all possess an equal likelihood of rupture. For example, in 70% of patients with AMI, multiple TCFA’s are found without rupture at sites remote from the culprit plaque, and in ~20% of cases plaque rupture is observed in necrotic core (NC) lesions with thicker fibrous caps (>100µm) or calcific nodules. These findings call into question a detection paradigm that relies solely on morphologic criteria and highlight the need to augment morphologic findings with critical biomechanical metrics, in order to accurately assess the risk of plaque rupture.
During the pathogenesis of atherosclerosis, the mechanical properties of the plaque are altered by a complex milieu of hemodynamic and biochemical processes. The ultimate event of plaque rupture is a biomechanical failure that occurs when a plaque with severely compromised mechanical properties is unable to withstand loads exerted on it. Therefore, in order to identify plaques with the highest risk of rupture, it is essential to complement morphologic information provided by current technologies with quantitative indices of mechanical failure. Our lab is developing a multi-modal paradigm to predict the risk of plaque rupture leading to AMI. Our paradigm will complement microstructural information obtained from OCT and plaque burden measurements from IVUS with mechanical measurements obtained using intracoronary laser speckle rheology (LSR). We envision the accurate prediction of coronary thrombosis will enable the development of new therapeutic devices and treatments to stabilize vulnerable plaque and reduce the incidence of AMI in the future.
Laser Speckle Rheology (LSR) of coronary atherosclerosis
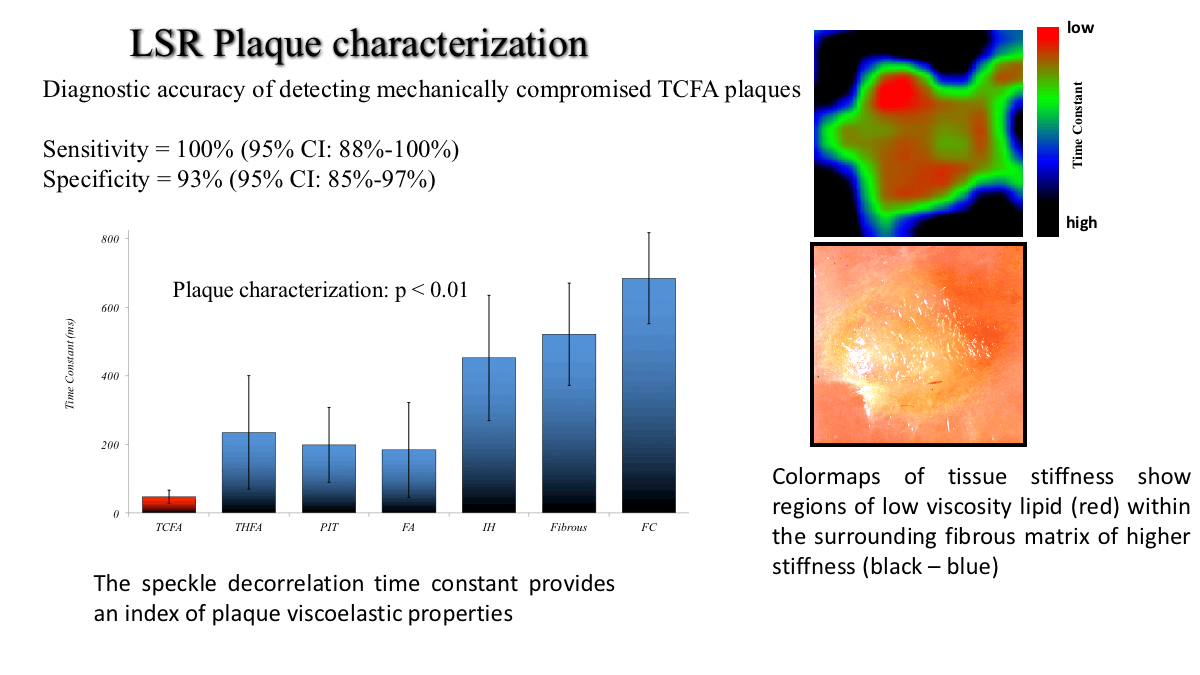 ILSR measures the mechanical properties of atherosclerotic plaques by evaluating the time-scale of intensity fluctuations from time-varying laser speckle patterns. In a viscoelastic medium, suspended particles undergo Brownian motion, which is directly related to the viscoelastic properties of the medium. Consequently, in TCFAs, because of the relatively low viscosity of the lipids, particles within a compliant necrotic core exhibit more rapid Brownian motion compared with stiffer fibrous regions of the plaque. Because scatterer motion causes a modulation of the laser speckle pattern, the measurement of temporal intensity variations provides information about the intrinsic viscoelastic properties of the plaque and can be used to determine plaque mechanical properties.
ILSR measures the mechanical properties of atherosclerotic plaques by evaluating the time-scale of intensity fluctuations from time-varying laser speckle patterns. In a viscoelastic medium, suspended particles undergo Brownian motion, which is directly related to the viscoelastic properties of the medium. Consequently, in TCFAs, because of the relatively low viscosity of the lipids, particles within a compliant necrotic core exhibit more rapid Brownian motion compared with stiffer fibrous regions of the plaque. Because scatterer motion causes a modulation of the laser speckle pattern, the measurement of temporal intensity variations provides information about the intrinsic viscoelastic properties of the plaque and can be used to determine plaque mechanical properties.
Intracoronary LSR
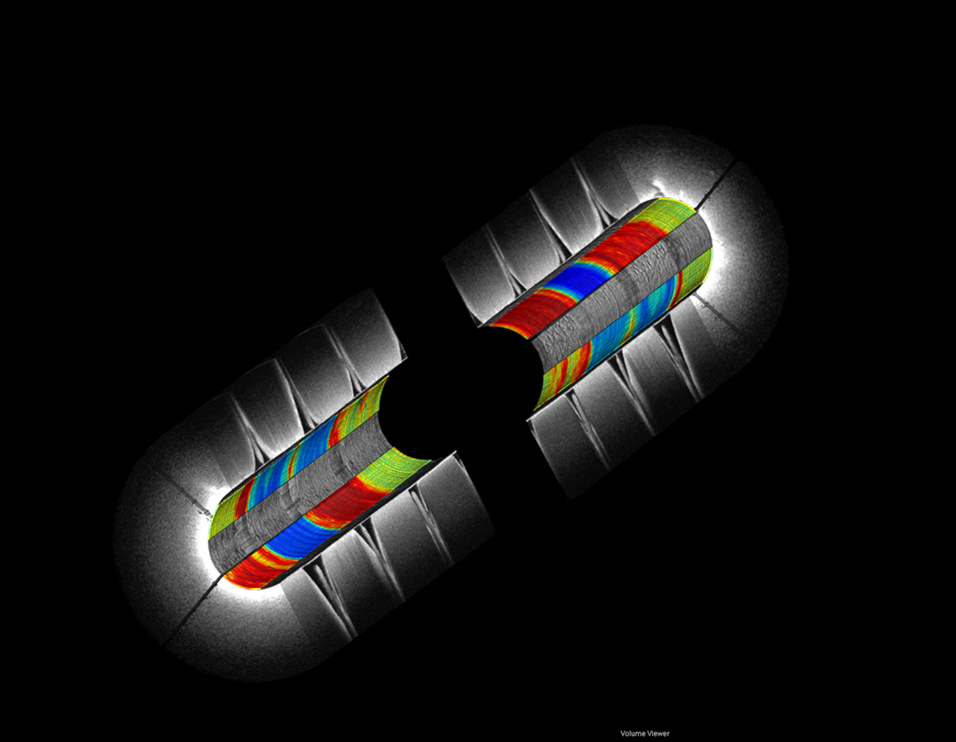
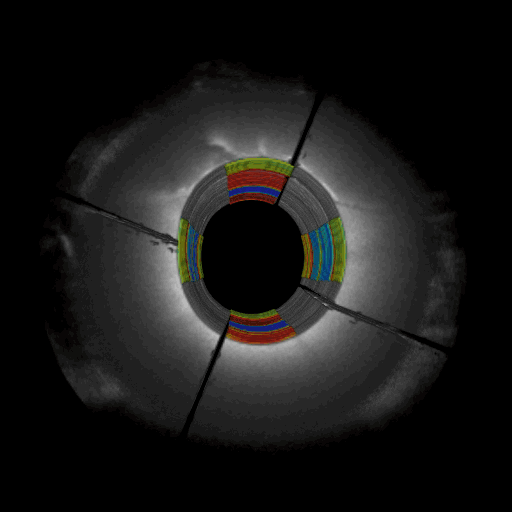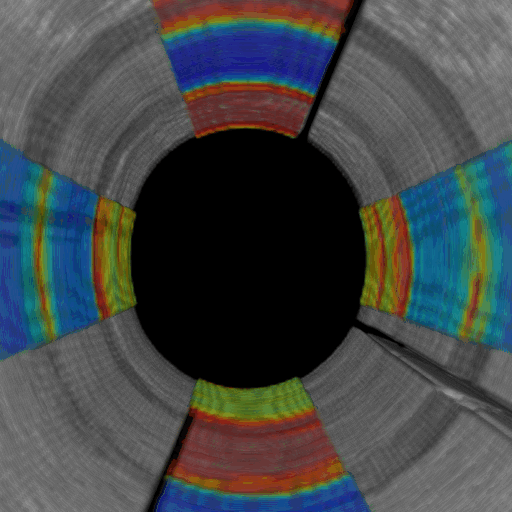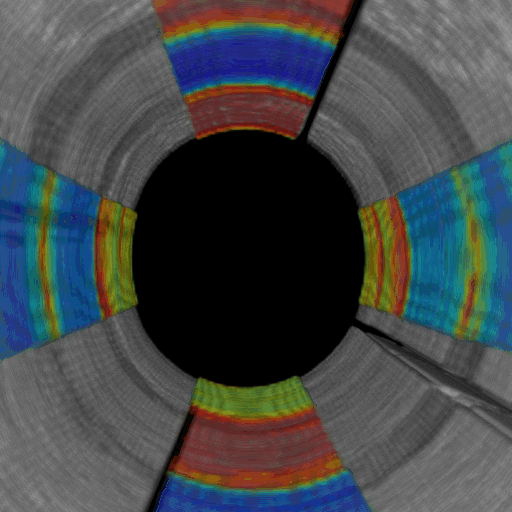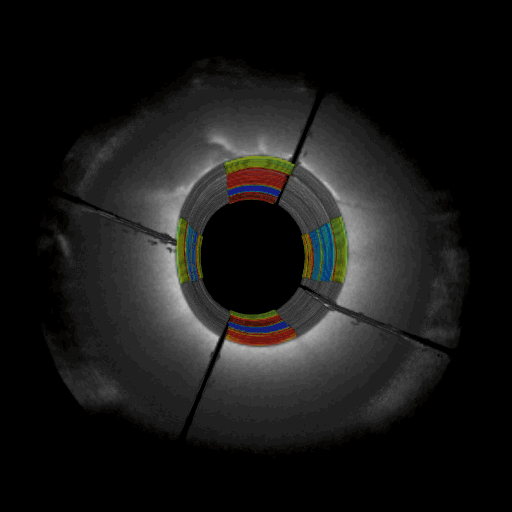
Omni-directional viewing through an intracoronary catheter: Cutaway and fly-through views of a coronary test phantom evaluated using intracoronary LSR. The colors represent spatially-varying mechanical properties (blue – red : soft – stiff)
Omni-directional catheter assembly
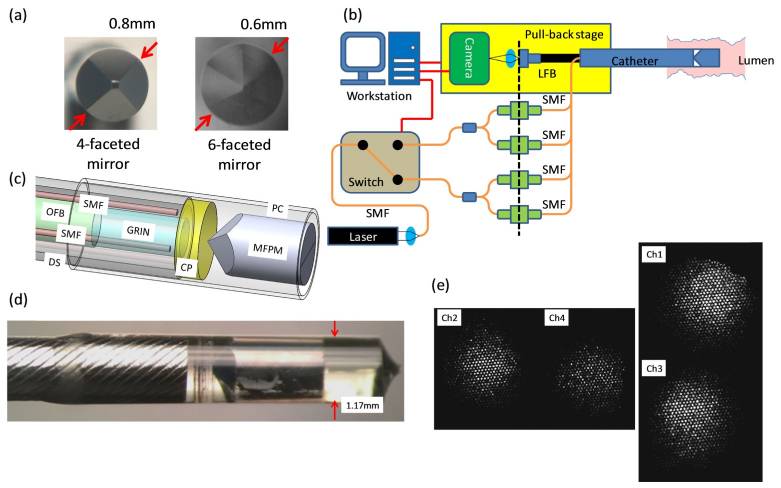
Our lab is developing miniature catheters and endoscopes to conduct LSR through luminal organs and vessels. The figure above shows the omni-directional LSR catheter assembly that includes miniature multi-faceted mirrors in (a). (b) The schematic diagram of the omni-directional LSR catheter, pull-back assembly and console hardware. Laser light (633 nm, 22.5mW), coupled into a single mode fiber (SMF) passes through a MEMS switch, and is split to 4 illumination fibers. The speckle patterns obtained from the lumen wall are transmitted through an optical fiber bundle (OFB) and are imaged on the CMOS camera. (c) The computer aided drawing shows the catheter distal optics assembly which incorporates single mode fibers (SMFs) for illumination, a circular polarizer (CP), a multi-faceted pyramidal mirror (MFPM), a gradient-index (GRIN) lens, and an optical fiber bundle (OFB). The distal assembly is housed within a protective polycarbonate (PC) tube and a customized drive shaft (DS). (d) The photograph of the distal optic assembly. The catheter diameter is 1.2mm. (e) Representative speckle images obtained from 4 catheter channels (mirror facets), with two opposite channels acquired simultaneously.
Relevant Publications
Intraluminal laser speckle rheology using an omni-directional viewing catheter. Wang J, Hosoda M, Tshikudi DM, Hajjarian Z, Nadkarni SK. Biomed Opt Express. 2016 Dec 8;8(1):137-150. doi: 10.1364/BOE.8.000137. eCollection 2017 Jan 1. PMID: 28101407
Optimizing flushing parameters in intracoronary optical coherence tomography: an in vivo swine study. Suter MJ, Kashiwagi M, Gallagher KA, Nadkarni SK, Asanani N, Tanaka A, Conditt GB, Tellez A, Milewski K, Kaluza GL, Granada JF, Bouma BE, Tearney GJ. Int J Cardiovasc Imaging. 2015 Aug;31(6):1097-106. doi: 10.1007/s10554-015-0668-0. Epub 2015 Apr 29. PMID: 25922149
A parametric study of inflammatory effects on plaque mechanical stress. Pei X, Nadkarni S, Li ZY. Int J Cardiol. 2016 Feb 15;205:157-159. doi: 10.1016/j.ijcard.2015.12.019.
The influence of optical fiber bundle parameters on the transmission of laser speckle patterns. Wang J, Nadkarni SK. Opt Express. 2014 Apr 21;22(8):8908-18. doi: 10.1364/OE.22.008908. PMID: 24787780
Optical measurement of arterial mechanical properties: from atherosclerotic plaque initiation to rupture. Nadkarni SK. J Biomed Opt. 2013 Dec;18(12):121507. doi: 10.1117/1.JBO.18.12.121507. Review. PMID: 24296995
Intravascular optical imaging technology for investigating the coronary artery. Suter MJ, Nadkarni SK, Weisz G, Tanaka A, Jaffer FA, Bouma BE, Tearney GJ. JACC Cardiovasc Imaging. 2011 Sep;4(9):1022-39. doi: 10.1016/j.jcmg.2011.03.020. Review. PMID: 21920342
Intravascular laser speckle imaging catheter for the mechanical evaluation of the arterial wall. Hajjarian Z, Xi J, Jaffer FA, Tearney GJ, Nadkarni SK. J Biomed Opt. 2011 Feb;16(2):026005. doi: 10.1117/1.3533322. PMID: 21361689
Laser speckle imaging of atherosclerotic plaques through optical fiber bundles. Nadkarni SK, Bouma BE, Yelin D, Gulati A, Tearney GJ. J Biomed Opt. 2008 Sep-Oct;13(5):054016. doi: 10.1117/1.2982529. PMID: 19021396
Evaluation of collagen in atherosclerotic plaques: the use of two coherent laser-based imaging methods. Nadkarni SK, Bouma BE, de Boer J, Tearney GJ. Lasers Med Sci. 2009 May;24(3):439-45. doi: 10.1007/s10103-007-0535-x. Epub 2008 Apr 2. Review. PMID: 18386093
Measurement of fibrous cap thickness in atherosclerotic plaques by spatiotemporal analysis of laser speckle images. Nadkarni SK, Bilenca A, Bouma BE, Tearney GJ. J Biomed Opt. 2006 Mar-Apr;11(2):021006. PMID: 16674181
Characterization of atherosclerotic plaques by laser speckle imaging. Nadkarni SK, Bouma BE, Helg T, Chan R, Halpern E, Chau A, Minsky MS, Motz JT, Houser SL, Tearney GJ. Circulation. 2005 Aug 9;112(6):885-92. Epub 2005 Aug 1. PMID: 16061738
OCT-based arterial elastography: robust estimation exploiting tissue biomechanics. Chan R, Chau A, Karl W, Nadkarni S, Khalil A, Iftimia N, Shishkov M, Tearney G, Kaazempur-Mofrad M, Bouma B. Opt Express. 2004 Sep 20;12(19):4558-72. PMID: 19484007