Laboratory for Optical Micromechanics and Imaging
Wellman Center for Photomedicine, Harvard Medical School, Massachusetts General Hospital






Laboratory for
Micromechanics and Imaging



about us
innovation and investigation of light-based technologies
At the Laboratory for Optical Micromechanics and Imaging, directed by Dr. Seemantini Nadkarni, the major thrust of our research is the innovation and investigation of light-based technologies that interrogate the mechanical behavior of tissue, and on shedding light on the role of tissue mechanics in disease etiology, progression and prognosis. Our laboratory is located at the Wellman Center for Photomedicine at Massachusetts General Hospital, Harvard Medical School. The Wellman Center is the world’s largest academic research facility dedicated to the development of light-mediated, diagnostic and therapeutic technologies. The research efforts in our laboratory are directed towards both, fundamental and translational areas of Biomedical Optics to address major questions related to clinical medicine and biological sciences. Currently, our research activities are focused on five major areas of investigation.
Main Research
Exploring The Possibilities

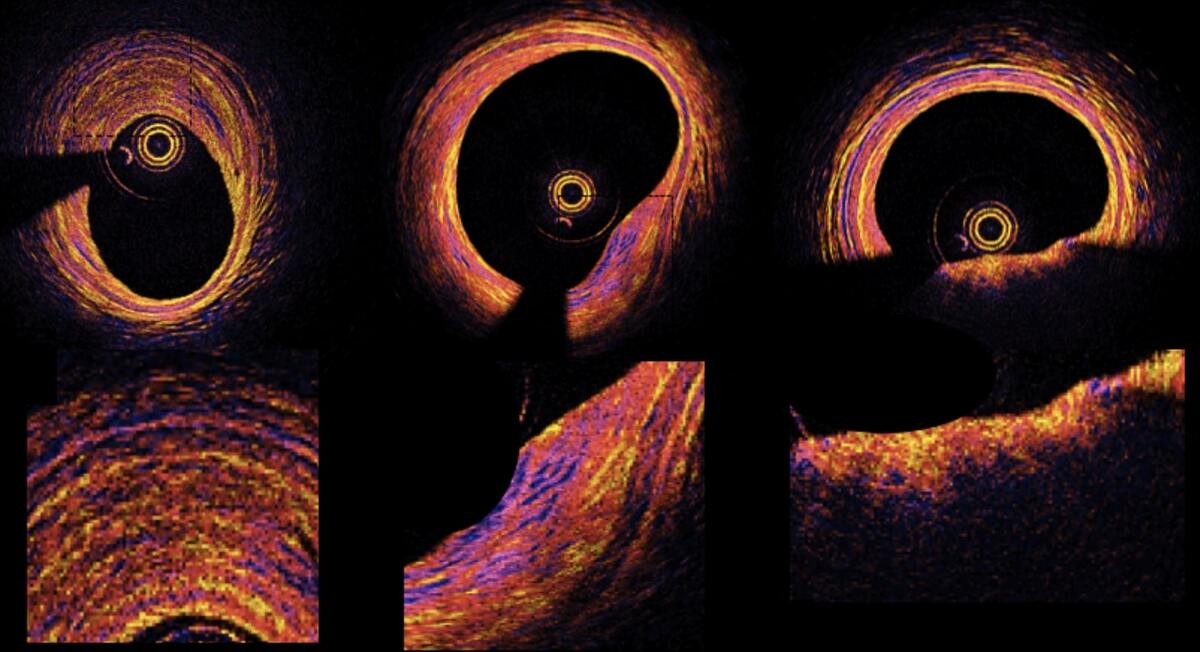
Polarization-Sensitive Optical Frequency Domain Imaging (PS-OFDI)
Intracoronary optical coherence tomography (OCT) and its next-generation counterpart, OFDI, have opened the exciting opportunity to visualize coronary atherosclerosis with exquisite micro-structural detail...
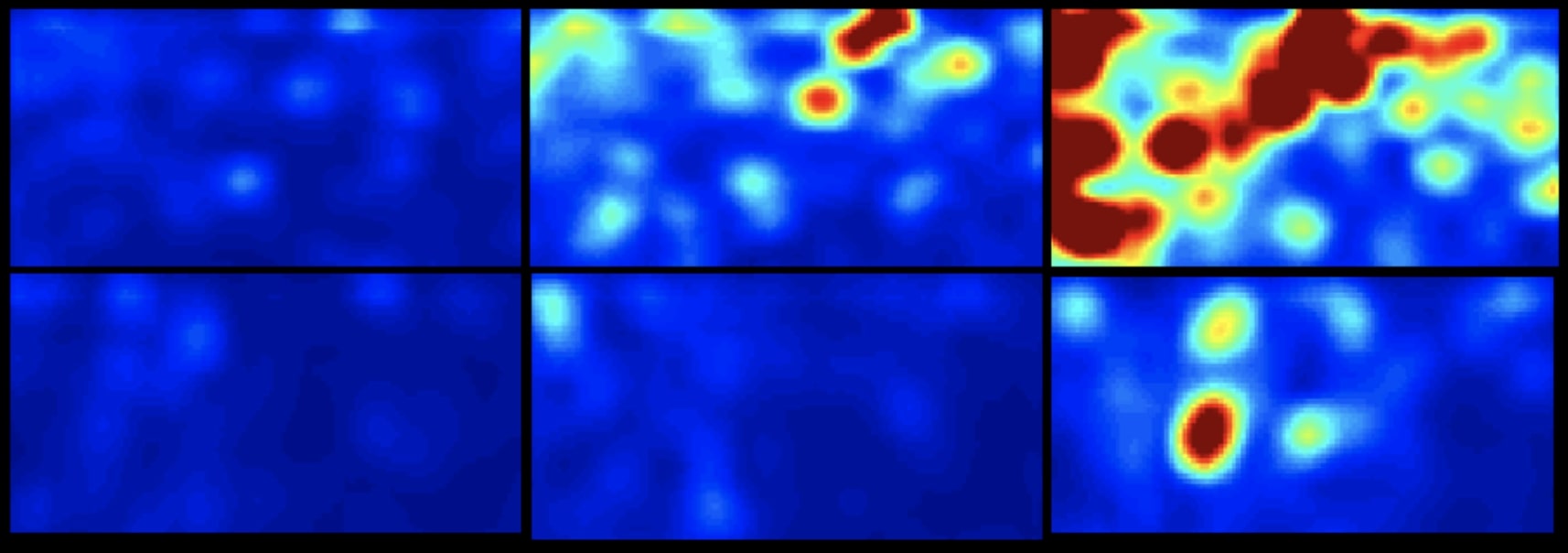
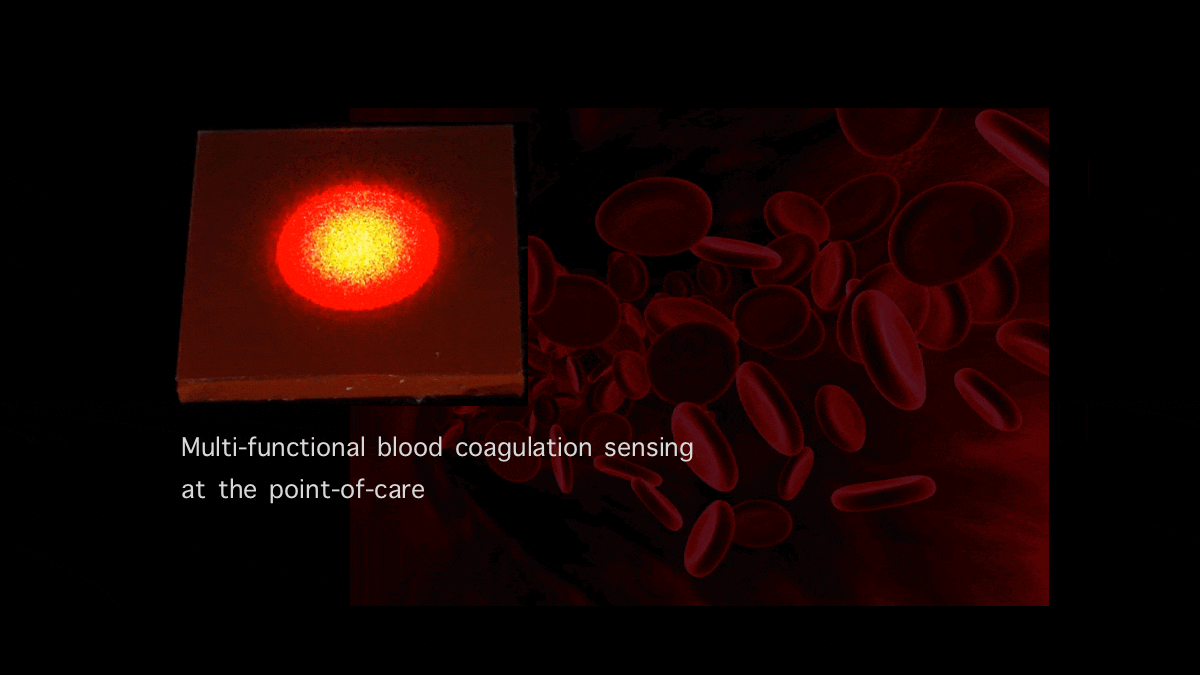
Blood Coagulation Sensing and in Vitro Diagnostics
Our team is developing new optical sensors that can quantify and report blood coagulation and platelet function profiles of patients within minutes using a drop of blood...
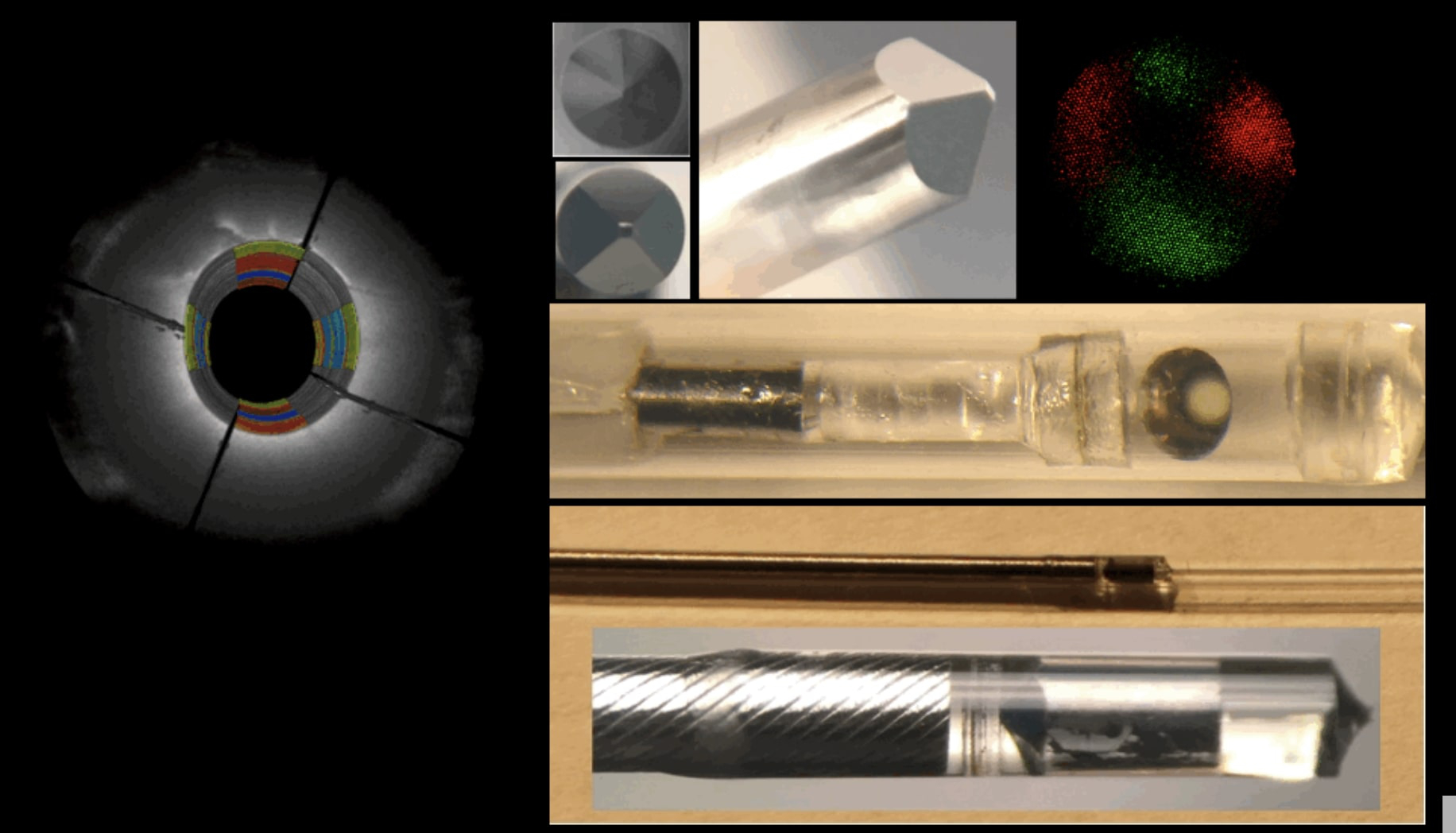
Intracoronary Plaque Biomechanics
Our laboratory has pioneered the development of a new technology, Intracoronary Laser Speckle Rheology (ILSR), for identifying mechanically unstable coronary plaques that cause myocardial infarction, the leading cause of death worldwide...

Laser Speckle Microrheology (LSM)
Our laboratory is leading the innovation of a new optical platform for light-based mechanical testing of tissue...

Extracellular Matrix Mechanics
Disease progression in tissues, from normal to pathological states, is often accompanied by changes in the intrinsic mechanical properties of the extracellular matrix (ECM)...